The effects of free radicals in an organism can be beneficial as well as adverse. Specialized cells such as macrophages utilize oxygen radicals for the immune system (i.e., as a defense reaction to pathogenic microorganisms). This is essential to life and health. The activities of external free radicals can also be useful: under the influence of UV-radiation, the hydroxyl radical formed from ozone and water in the atmosphere plays an important role in combating air pollution, for instance. But free radicals can also damage the body. When present in high concentrations (oxidative stress) they attack important metabolic proteins, cell membranes and even DNA and change their characteristics, which can lead to damage among various types cells of the body. Hence, oxidative stress facilitates the incidence and progression of atherosclerosis. Consequently vascular diseases like heart attack and stroke through the increased oxidation of lipids and damage to the internal cell lining of blood vessels (the endothelium) (1). In addition to numerous chronic diseases, normal signs of aging have been linked to progressive oxidative processes and the gene mutations they trigger – in particular in mitochondrial DNA. In the worst case scenario the result is uncontrolled cell proliferation (cancer).
To protect itself against cell damage due to free oxygen radicals, the body possesses an arsenal of highly potent enzymes (e.g., superoxide dismutase) that capture the aggressive molecules and neutralize them (i.e., they exhibit “ antioxidant ” activity). The functionality of these proteins depends on several essential trace elements (e.g., zinc). Apart from these enzymes the organism has at its disposal for defense fat-soluble coenzyme Q10, for instance, the production of which diminishes with increasing age, as well as other proteins and alpha-lipoic acid. In addition to these physiological systems, the network of antioxidants includes various substances that can be supplied to the organism: the essential vitamins C and E, requirements of which are well documented; polyphenols (e.g., flavonoids), requirements of which are comparatively poorly documented, since there are no known deficiency diseases; and carotenoids (e.g., beta-carotene), which are not true antioxidants but can absorb the energy of free radicals. Thanks to their structure these molecules exhibit various solvent properties and are therefore able to neutralize radicals, functioning as a network in all compartments of the cell – in both aqueous and lipid milieus. Ideally the unavoidable occurrence of free radicals is a balanced by the protective effect of antioxidants synthesized by the body and ingested in the diet (oxidative balance).
 It is evident that in the longer term the
It is evident that in the longer term the  The effects of free radicals
The effects of free radicals Testing the preventive effects of antioxidants in studies
Testing the preventive effects of antioxidants in studies Results from epidemiological studies
Results from epidemiological studies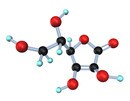 Vitamin C
Vitamin C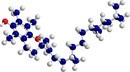 Vitamin E
Vitamin E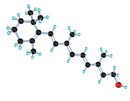 Beta-carotene
Beta-carotene Meta-analyses
Meta-analyses